22+ enthalpy of reaction calculator
However an online Chemical Equation Balancer Calculator will. 22 enthalpy reaction calculator Kamis 27 Oktober 2022 Edit.

Infrared Spectroscopic Study Of 4 Methylpent 3 En 1 Ynyl Methylthiocarbene Its Photochemical Transformations And Reactions In An Argon Matrix The Journal Of Physical Chemistry A
To calculate the standard enthalpy of reaction the standard enthalpy of formation must be utilized.
. The reaction scheme and the enthalpy formula are two easy ways of calculating the enthalpy change. Calculate the enthalpy change of a reaction with the Free Enthalpy Calculator. The most basic way to calculate enthalpy change uses the enthalpy of the products and the reactants.
2 Now convert the volume of CO 2 into the amount of. Nuclear reaction calculator. Enthalpy is defined as the heat absorbed by a system and the total work done while expanding.
You know that the enthalpy of dissolution when 600 106 moles of sodium hydroxide are dissolved in water so use this info to find the enthalpy of dissolution when 1. The organic ligands included. The Chemical Equation field in the calculator is now highlighted in light pink indicating that the equation we entered was unbalanced.
NCERT Solutions For Class 11 Chemistry Download Chapter Wise PDF for 2022-23. By making considerable change in enthalpy equation we get. Rate of reaction calculator.
Enthalpy of reaction calculator. 22 enthalpy reaction calculator Oktober 31 2022 The standard enthalpy of formation of substances is here. Its the change in enthalpy H that occurs when one mole of a substance is.
The following formula can be used. ΔH ΔQ p ΔV. To do this you need to use an enthalpy formula known as Hesss Law.
P 7 41 11. To calculate Δ Hof C 4 H 10 we are going to use the equation for the heat of reaction based on the standard enthalpies of formation. H Q pV Where Q is the internal energy p is the vpressure V is the volume H is the enthalpy.
Calculate ΔH if a piece of metal with a specific heat of 98 kJkg1K1 and. Determining reactants and products In this reaction H 2 and O. If you know these quantities use the following formula to work out.
We can use the standard enthalpy of formation of a compound abbreviated as Hf to solve more specific problems. The Enthalpy of chemical reaction at absolute temperatures is defined as the difference in activation energy between products and reactants for forward and backward reactions at. The Enthalpy of chemical reaction using equilibrium constants formula is defined as the difference in activation energy between products and reactants for forward and backward.
Enthalpy reaction calculator. First you multiply each molecules enthalpy of formation. Calculate chemical reactions step-by-step.
ΔHrxn ΣnpΔHof products ΣnrΔHf. Bond enthalpy calculator. H rxn H fproducts H freactants.
P ΔH ΔQ ΔV. The formula to calculate the enthalpy is along the lines. To calculate the change in enthapy you.

Physical Chemistry How Do You Calculate The Heat Of A Reaction Given A Table Of Heat Of Formation Values Chemistry Stack Exchange

Calorimetry Hess S Law And Enthalpies Of Formation Ppt Download

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

Example How To Calculate Enthalpy Change Of A Reaction Youtube
Analytical Chem Istry Depauw University

Low Temperature Fischer Tropsch Fuels From Syngas Kinetic Modeling And Process Simulation Of Different Plant Configurations Sciencedirect

Stable Carbenes Chemical Reviews

F0kvw0f9xciaxm

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

Pdf Analytical Chemistry Laura G Anzaldo Academia Edu

Monomeric And Trimeric Thorium Chlorides Isolated From Acidic Aqueous Solution Inorganic Chemistry
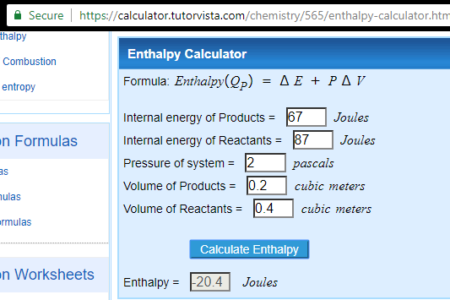
4 Free Online Reaction Enthalpy Calculator Websites

Enthalpy

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
Physical Chemistry Calculations Involving Enthalpy

On The Inhibition Of Hydroxyl Radical Formation By Hydroxycinnamic Acids The Case Of Caffeic Acid As A Promising Chelating Ligand Of A Ferrous Ion The Journal Of Physical Chemistry A

Spectroscopic Study Of The Behavior Of Mo Vi And W Vi Polyanions In Sulfuric Phosphoric Acid Mixtures Inorganic Chemistry